Lohocla Research Development Pipeline
Kindolor: the network approach to treating chronic pain
Normally, pain is one of the survival responses that protects us against further harm from environmental hazards. Accordingly, the systems for sensing painful stimuli, conducting painful sensations, and interpreting those sensations have evolved to be resistant to the failure of any single component of the pain sensory systems. Even complete loss of function in one contributing system, such as an ion channel involved in the conduction of pain information from the periphery to the central nervous system (CNS), can affect some types of pain but not others. Ion channels represent a common target for analgesic medications. However, even in the case of the pain sensation that involves the participation of a particular ion channel, researchers have realized that pain consists of changes in several systems in addition to the ion channel in question.
Despite the fact that the complexity and redundancy of the pain sensory and conducting systems have been well described by physiologists, biochemists, and molecular biologists, these qualities seem to be forgotten in the development of novel pharmaceuticals for treatment of pain in general, but particularly in the case of treatments for chronic pain.
Distinct from acute pain associated with injury, chronic pain involves another level of complexity. With chronic pain, the relevant sensory, conducting, and interpreting systems change function (either adapting or maladapting) during the process of development of chronic pain conditions. These conditions include cancer pain, post-surgical and post-traumatic pain, neuropathic pain, inflammatory pain, headache, orofacial pain, visceral pain, and musculoskeletal pain. Moreover, within these conditions lie a plethora of specific categories such as painful diabetic neuropathy (PDN) or fibromyalgia, and each of these subtypes result from particular etiologies. Luckily, while the systems that sense pain signals and carry these signals to the brain are complex, they are not infinitely different and are organized into discernible networks.
Network models suggest that partial inhibition of a surprisingly small number of targets can be more efficient than the complete inhibition of a single target.
—Csermely et al., 2005
In contemplating a novel approach to developing a pharmaceutical for the treatment of chronic pain, Lohocla Research established the following goals.
- The medication must act on the early stages of the pain sensory process before being distributed throughout the nervous system, i.e., on the peripheral sensory neurons that transmit the pain information to the CNS.
- The medication should not penetrate into the CNS. Avoiding interaction with the CNS prevents addiction and unwanted side effects generated by drugs that act on the CNS (e.g., cognitive effects, confusion, and respiratory suppression).
- Create a poly-target molecule that has an overall additive or synergistic effect by simultaneously acting selectively on more than one relevant target in the peripheral sensory system for pain, thereby overcoming the multiplicity and redundancy in the pain-generating sensory system.
Lohocla Research selected the targets in the sensory neurons that had the greatest evidence for changing (adapting or maladapting) during the development of chronic pain. These systems included the peripheral NMDA receptors, and the sensory neuron sodium ion channels Nav1.7 and Nav1.8. Located outside the brain and spinal cord, all of these targets are primarily found on sensory neurons and are upregulated with development of particular chronic pain syndromes. The upregulation of NMDA receptors and sodium channels is maladaptive. In response, Lohocla Research designed and synthesized Kindolor, a molecule that acts as an antagonist (or inhibitor) at NMDA receptors and the relevant voltage-sensitive sodium channels.
Lohocla Research Corporation rationally designed a novel small molecule that we named Kindolor for treatment of chronic pain syndromes. The molecule reduces the over-activity of the nociceptive (pain-sensing) peripheral neural network that generates chronic pain. Kindolor has negligible penetrance into the brain, thus reducing deleterious CNS side effects. Accordingly, Kindolor’s actions are confined to the peripheral nervous system, where Kindolor has been shown to produce significant anti-hyperalgesic effects in six animal models of chronic pain. No genotoxic effects of Kindolor have been noted. Acute dosing levels 20 to 30 times higher than doses producing therapeutic effects produced no overt signs of toxicity, and we estimate the therapeutic index for Kindolor to be greater than 30.
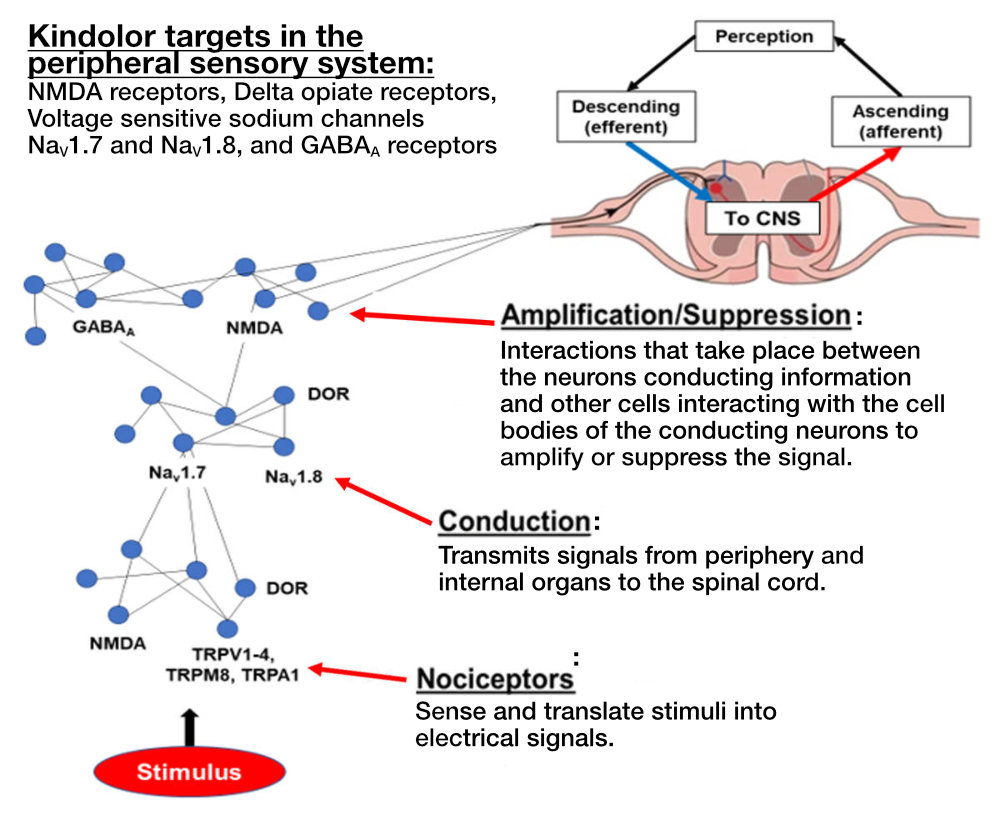
Background
Over 100 million adults in the U.S. suffer from intermittent or constant chronic pain, and chronic pain affects at least 10 percent of the world’s population. The primary pharmaceuticals for treatment of chronic pain have been natural or synthetic opioids. However, the use of opioids for pain treatment has resulted in an “epidemic” of opioid abuse, addiction, and lethal overdoses. Through a process of rational drug design, Lohocla Research has generated a new chemical entity (NCE) called Kindolor. Kindolor is a non-opiate, non-addicting molecule that was developed specifically to target the aberrant over-activity of the peripheral sensory system that is integral in the initiation and propagation of chronic pain. Kindolor dampens the over-activity of the signaling network that conducts and magnifies pain signals (Fig. A), but leaves the normal response to acute painful stimuli intact. Lohocla Research has developed a process to synthesize Kindolor at 99 percent purity. Pre-clinical studies have demonstrated the efficacy of Kindolor to reduce or eliminate chronic pain generated in five animal models at doses compatible with use of Kindolor in humans. Initial evidence demonstrates the safety (high TI) of Kindolor as well as its uneventful metabolism. Additional attractive features of Kindolor are that it can prevent the development of chronic pain if given soon after tissue injury. Additionally, if combined with low doses of mu opioid receptor agonists, Kindolor produces a substantial “opiate sparing” effect through synergistic actions with the opiates. Lohocla Research is working to bring Kindolor to the public. An IND application is in process, and Phase 1 trials are planned for 2023, followed by Phase 2 trials for efficacy in treating diabetic neuropathy. In all, our goal is to license to or partner with a Pharma company willing and able to bring this innovative medication to the chronic pain sufferer.
Public Health Relevance Statement
The U.S. and other countries around the world are facing “dual crises of pain and opioid addiction.” Lohocla Research Corporation has responded to the opioid crisis and medication development challenge by designing, synthesizing, and demonstrating, in pre-clinical studies, the efficacy and safety of a non-opiate, non-addictive new chemical entity (NCE) for treatment of chronic pain. This NCE, called Kindolor, has significant additional benefits of being able to prevent the development of chronic pain if administered soon after tissue injury, including post-operative conditions. Kindolor also has a highly significant “opiate sparing” effect in conditions that may require the use of opiates, since Kindolor demonstrates a strong synergistic effect with morphine. Given the FDA approval of our IND status, we will complete first-in-human, Phase I clinical studies for safety and Phase 2a studies of efficacy to bring our medication to chronic pain sufferers.

